What is VivitraTM (Trastuzumab)?
What is VivitraTM (Trastuzumab)?
VivitraTM (Trastuzumab) is a highly biosimilar medicinal product of reference biologic. It is a humanized monoclonal anti-HER2 (Human epidermal growth factor receptor 2) antibody. It comprises of 1328-amino acids; glycoprotein.1
 VivitraTM (Trastuzumab) is indicated in which condition?
VivitraTM (Trastuzumab) is indicated in which condition?
Metastatic Breast Cancer (MBC): VivitraTM (Trastuzumab) is indicated for the treatment of patients with HER2 overexpressing metastatic breast cancer. VivitraTM (Trastuzumab) is also indicated in combination with aromatase inhibitor for the treatment of patients with HER2 overexpressing and hormone receptor-positive metastatic breast cancer.1
Early Breast Cancer (EBC): VivitraTM (Trastuzumab) is indicated for the treatment of patients with HER2 overexpressing early breast cancer following surgery, chemotherapy (neo adjuvant or adjuvant) and radiotherapy (if applicable). VivitraTM (Trastuzumab) is also indicated for adjuvant treatment of patients with HER2 overexpressing node positive or node negative breast cancer i) as part of treatment regimen comprising doxorubicin, cyclophosphamide, and either paclitaxel or docetaxel ii) with docetaxel and carboplatin.1
Metastatic Gastric Cancer (MGC): VivitraTM (Trastuzumab) is indicated for the treatment of patients with HER2 overexpressing metastatic gastric or gastroesophageal junction adenocarcinoma in combination with capecitabine or 5- fluorouracil and cisplatin who have not received prior anti-cancer treatment for their metastatic disease.1
 How does VivitraTM (Trastuzumab) work?
How does VivitraTM (Trastuzumab) work?
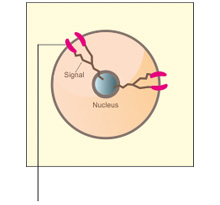
- The HER2 gene encodes the receptor tyrosine kinase HER2 and is often over-expressed in breast cancer and gastric cancer.2,3 Increased number of HER2 contributes to tumor progression.2
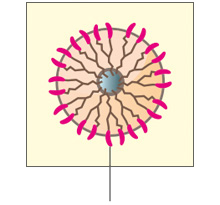
- Breast cancers can have up to 25–50 copies of the HER2 gene and up to 40–100 fold increase in HER2 protein resulting in 2 million receptors expressed at the tumor cell surface.4
- Increased HER2 signaling plays a role in increasing proliferation and survival of the primary tumor and distant lesions which upon completion of full transformation cause metastases.2
- About 1 out of 5 of gastric cancers has too much of a growth-promoting protein called HER2/neu (or just HER2) on the surface of the cancer cells. Tumors with increased levels of HER2 are called HER2positive- HER2 +ve.3
- Approximately 1 in 5 patients with breast cancer has HER2-positive breast cancer.4
A. Normal cell
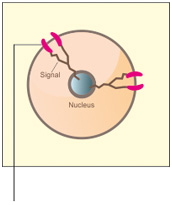
Normal HER2 receptors send signal to grow and divide normally
B. Abnormal HER2 positive cancer cell
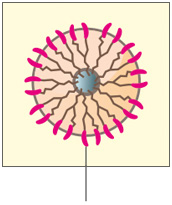
Too many HER2 receptors send more signals causing cells to grow too quickly.
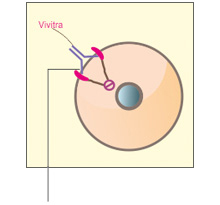
C. How VivitraTM (Trastuzumab) may work
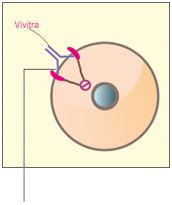
VivitraTM (Trastuzumab) may stop the HER2 receptors from signaling the cells to grow.1 VivitraTM (Trastuzumab) may be able to flag tumor cells for destruction by the immune system-NK (Natural Killer) cells1
Figure: HER2 normal (A) / Overexpression (B) / Vivitra blocks HER2(C)
 HER2 testing before VivitraTM (Trastuzumab) therapy is required, why?
HER2 testing before VivitraTM (Trastuzumab) therapy is required, why?
- HER2 testing is mandatory before starting Vivitra TM (Trastuzumab) therapy.1
- HER2 testing is performed with the tumor sample removed during surgery or using a needle.
- Having too many HER2 receptors may make the cancer grow and divide faster, creating more HER2-positive cancer cells.
- IHC Test - Immunohistochemistry test - Cancer cells are examined under a microscope to see whether there is too much HER2 protein present on the cell surface.3,5
- FISH Test – Fluoroscence insitu hybridization test- The cell nucleus controls cell growth and reproduction. FISH testing measures the number of copies of the HER2 gene in the nucleus.3,5
- The pathologist will interpret the results and score the patient as HER2-positive, HER2-negative, or equivocal (which means that the tumor is considered neither HER2-positive nor HER2-negative).
- IHC test results are scored as negative (0 or 1+), equivocal (2+), or positive (3+).3,5
- FISH test results are scored as negative, equivocal, or positive.3,5
 VivitraTM (Trastuzumab) Dosage and Administration1
VivitraTM (Trastuzumab) Dosage and Administration1
- VivitraTM (Trastuzumab) is given by an IV (intravenous) infusion, which means that it is given through a needle inserted into your vein.
- You will most likely continue to take VivitraTM (Trastuzumab) until your disease grows or spreads, or until the side effects become unmanageable.
Cycle |
Dose |
Route/Duration |
Weekly |
||
Loading dose |
4 mg/kg |
I.V. infusion over 90 minutes |
Subsequent dose |
2 mg/kg |
I.V. infusion over 30 minutes |
3 Weekly |
||
Loading dose |
8 mg/kg |
I.V. infusion over 90 minutes |
Sub sequent dose |
6 mg/kg |
I.V. infusion over 30 minutes |
 References
References
- VivitraTM (Trastuzumab) Prescribing Information
- Freudenberg JA, Wang Q, Katsumata M, Drebin J, Nagatomo I, Greene MI. The role of HER2 in early breast cancer metastasis and the origins of resistance to HER2-targeted therapies. Exp Mol Pathol. 2009 Aug;87(1):1-11
- Stomach cancer American cancer society 2013 Available at www.cancer.org/stomach-cancer-pdf Accessed november 2015
- Gutierrez C, Schiff R. HER2: biology, detection, and clinical implications. Arch Pathol Lab Med. 2011 Jan;135(1):55-62
- ASCO 2013 Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF; American Society of Clinical Oncology; College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013 Nov 1;31(31):3997-4013