VivitraTM (Trastuzumab) is a humanized monoclonal anti-HER2 antibody. It has been expressed in Chinese Hamster Ovary (CHO) cell line. It comprises of 1328-amino acids; glycoprotein. It has molecular weight of 148 kDa (approx.)1
 Mechanism of Action
Mechanism of Action
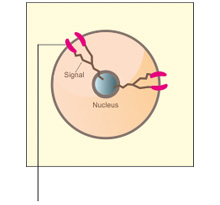
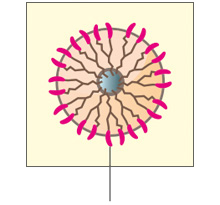
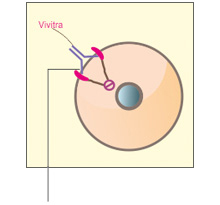
VivitraTM (Trastuzumab) binds with high affinity and specificity to an epitope located in the extracellular sub-domain IV of HER2 present juxtaposed to the cell membrane region. Binding of VivitraTM (Trastuzumab) to HER2 inhibits HER2 signalling and prevents the proteolytic cleavage of its extracellular domain, leading to an inhibition of proliferation of HER2 overexpressing human tumor cells.1
VivitraTM (Trastuzumab) is also known to carry out its function via an immune effector mechanism, ADCC (antibody-dependent cell-mediated cytotoxicity), by binding to HER2 overexpressing cancer cells in the presence of NK(natural killer) cells.1
 Indications
Indications
Metastatic Breast Cancer (MBC):
VivitraTM (Trastuzumab) is indicated for the treatment of patients with HER2 overexpressing metastatic breast cancer. VivitraTM (Trastuzumab) is also indicated in combination with aromatase inhibitor for the treatment of patients with HER2 overexpressing and hormone receptor-positive metastatic breast cancer.
Early Breast Cancer (EBC):
VivitraTM (Trastuzumab) is indicated for the treatment of patients with HER2 overexpressing early breast cancer following surgery, chemotherapy (neo adjuvant or adjuvant) and radiotherapy (if applicable). VivitraTM (Trastuzumab) is also indicated for adjuvant treatment of patients with HER2 overexpressing node positive or node negative breast cancer (i) as part of treatment regimen comprising doxorubicin, cyclophosphamide, and either paclitaxel or docetaxel (ii) with docetaxel and carboplatin.
Metastatic Gastric Cancer (MGC):
VivitraTM (Trastuzumab) is indicated for the treatment of patients with HER2 overexpressing metastatic gastric or gastroesophageal junction adenocarcinoma in combination with capecitabine or 5- fluorouracil and cisplatin who have not received prior anti-cancer treatment for their metastatic disease.
 Dosage and Administration1
Dosage and Administration1
Cycle |
Dose |
Route/Duration |
Weekly |
||
Loading dose |
4 mg/kg |
I.V. infusion over 90 minutes |
Subsequent dose |
2 mg/kg |
I.V. infusion over 30 minutes |
3 Weekly |
||
Loading dose |
8 mg/kg |
I.V. infusion over 90 minutes |
Subsequent dose |
6 mg/kg |
I.V. infusion over 30 minutes |
 Special Warnings and Precautions for Use1
Special Warnings and Precautions for Use1
Cardiomyopathy: VivitraTM (Trastuzumab) can result in sub-clinical and clinical cardiac failure manifesting as Congestive heart failure (CHF), and decreased Left ventricular ejection factor (LVEF), with greatest risk when administered concurrently with anthracyclines. Evaluate cardiac function prior to and during treatment. Discontinue VivitraTM (Trastuzumab) for cardiomyopathy.
Infusion reactions, Pulmonary toxicity: Discontinue VivitraTM (Trastuzumab) for anaphylaxis, angioedema, interstitial pneumonitis, or acute respiratory distress syndrome.
Embryo-Fetal Toxicity: Exposure to VivitraTM (Trastuzumab) during pregnancy can result in oligohydramnios, in some cases complicated by pulmonary hypoplasia and neonatal death.
Most Common Adverse Events with VivitraTM (Trastuzumab): These are anemia, neutropenia, thrombocytopenia, diarrhoea, nausea, vomiting, stomatitis, pyrexia, asthenia.
 References
References
- VivitraTM (Trastuzumab) Prescribing Information